Dubai has one of the biggest variety of escorts from different parts of the world. The city is a playground for sex tourist and if you have the money, you can find some very sexy ladies.The sex escort in dubai industry in Dubai is a lucrative business, allowing sex workers to make tens of thousands or even hundreds of thousands of euros. However, it is a highly dangerous industry, largely ignored by law enforcement.
Quality Throughout
Quality at Astrella Pharmaceuticalis clearly demonstrated in all phases of the product lifecycle. Quality supervision begins at the test facilities with careful documentation and general conduct of non-clinical safety studies. This ensures compliance with current Good Laboratory Practice (GLP) and consequently, the integrity of the data produced. Quality supervision then follows through clinical trials, production and distribution, and concludes with shelf-life surveillance.
Quality During Clinical Stages
During clinical stage development, quality supervision guarantees that the fundamentals of Good Manufacturing Practices (GMPs) are consistently applied. Quality is built into the different phases of clinical development to secure:
-
The safety and rights of our studies’ participants
-
The reliability of the data submitted to health Authorities
-
The complete adherence to all current Good Clinical Practices (GCPs)
Our clinical Quality Management System encompasses clinical research documents, activities and information
management systems. Moreover, Astrella Pharmaceutical ensures that quality is a main concern, not only in
our own processes and procedures, but also in those of our vendors, investigators and contractors who collaborate with us at the clinical stage.
Quality Throughout the Manufacturing Process
Quality at Astrella Pharmaceuticalis clearly demonstrated in all phases of the product lifecycle. Quality supervision begins at the test facilities with careful documentation and general conduct of non-clinical safety studies. This ensures compliance with current Good Laboratory Practice (GLP) and consequently, the integrity of the data produced. Quality supervision then follows through clinical trials, production and distribution, and concludes with shelf-life surveillance.
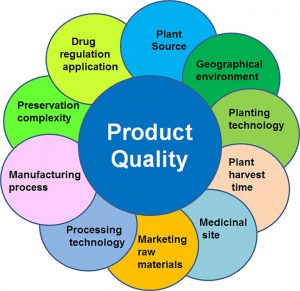
Quality During Production
Manufacturing processes are validated and equipment is tested and certified test methods are confirmed to ensure that each product is of reproducible quality
Continuous improvement practices are employed so that processes and procedures are continually updated.

Escort ny – Get to know beautiful escorts from the most reputable escort sites in NY. Browse profiles & call girls offering their services in your area 24/7!Medicare doesn’t escort ny cover escorts or home-care services. But nonprofit and private hospitals are starting to help patients find them.